Accreditations
UltraSlim® and UltraSmooth® have earned 12 international standards accreditations through independent laboratory testing, bench testing, and animal testing, including:
- IEC 60601-1:2012 reprint Medical Electrical Equipment - Part 1 General Requirements for Basic Safety and Essential Performance.
- IEC 60601-1-2:2007 Medical electrical equipment. General requirements for basic safety and essential performance. Collateral standard. Electromagnetic compatibility. Requirements and tests.
- IEC 60601-2-57:2011, Medical Electrical Equipment - Part 2-57: Particular requirements for the basic safety and essential performance of non-laser light source equipment intended for therapeutic, diagnostic, monitoring and cosmetic/aesthetics.
- ANSI/AAMI ES60601-1:2005/(R) 2012 and A1:2012, C1:2009/(R)2012 and A2:2010/(R)2012 - Part 1 General Requirements for Basic Safety and Essential Performance.
- IEC 62471:2006 Photobiological safety of lamps and lamp systems.
- ISTA 3A Shipping & Handling (ASTM) testing.
- Toxikon Animal Testing 14-00947-N1 Non-GLP Report.
- FDA Good Manufacturing Practices.
- FDA Clearances K160880, K150336, K180338, and K202361.
- FDA_GMP-Certified devices - the global standard for medical excellence.
Patents
We don’t just design and manufacture best-in-class medical devices for photobiomodulation. We invented and patented fat reduction and cellulite treatments using noncoherent light!
Today, UltraSlim® and UltraSmooth® are manufactured under license and subject to our 9 patents in 31 countries, including 4 utility patents granted by The United States Patent and Trademark Office:
- US patent 9,044,595, System and method for reducing lipid content of adipocytes in a body
- US patent 9,498,641, Fat reducing device and method utilizing optical emitters
- US patent 9,808,314, Fat reducing device and method utilizing optical emitters
- US patent 10,946,210, Cellulite and fat reducing device and method utilizing optical emitters
- US patent pending 63/074,790, Respiratory Treatment Device And Method Utilizing Optical Emitters
- European Union patent EU 14860875.5, Cellulite And Fat Reducing Device And Method Utilizing Optical Emitters
- Colombian patent NC2019/0004085, DISPOSITIVO Y MÉTODO PARA REDUCIR LA GRASA CORPORAL MEDIANTE LA UTILIZACIÓN DE EMISORES ÓPTICOS
- Mexican patent MX/a/2019/004642, DISPOSITIVO Y MÉTODO PARA REDUCIR LA GRASA CORPORAL MEDIANTE LA UTILIZACIÓN DE EMISORES ÓPTICOS
- Brazilian patent BR 11 2019 008187 9, MÉTODO DE REDUÇÃO DO CONTEÚDO DE LIPÍDIOS DE ADIPÓCITOS SUBCUTÂNEOS
Trademarks and Service Marks
The United States Patent and Trademark Office has awarded our devices registered trademarks and service marks UltraSlim® and UltraSmooth®, along with registered trademarks UltraSlim Cold Light®, Cellulize®, and Vevazz®.
Research
In 2011, our founder Terry J. Ward, Sr. M.H.A. made a quantum leap discovery in photobiomodulation and non-invasive medicine using a special type of non-laser light. Terry’s breakthrough inventions have since earned 9 patents in 31 countries.

In 2012, Terry assembled prototype UltraSlim® LED arrays that were 20,000 times more powerful than state-of-the-art Zerona® aesthetic lasers which were FDA-cleared for fat reduction in 2010. Shown at left is a porcine cadaver illuminated by Terry’s prototype. Show at right is the same porcine cadaver illuminated by a Zerona® laser:
By 2013, Terry had assembled working UltraSlim® devices. After considerable research, innovation, and testing, what is now Photonica USA emerged from start-up to become an international leader in the "medicine of light".
In July 2013, a Longevity Testing Lab was established at its R&D facilities in Cocoa Beach, on the technology-driven Space Coast of Florida. Today, 96% of those original medical lights are still operating properly after more than 98,000 hours of continuous use. The red beacon is visible from miles away and "The Red Room" became an established Cocoa Beach landmark.

In 2014, our research team passed independent laboratory testing and received ten international standards accreditations.
In February 2015, we were awarded our first FDA clearance (K150336) for skin treatments with the Photonica® Professional device.
A few months later, we received the first of four U.S. patents for fat reduction with noncoherent light and conducted the first IRB clinical trials (see NCT02867150 at ClinicalTrials.gov).
Based on those results, our second FDA clearance (K160880) was awarded in 2016 for immediate fat loss. Photonica® Professional was then rebranded as "UltraSlim®."
Initially branded as Cellulize® during pre-marketing research and development, UltraSmooth® received its first FDA clearance (K180338) in 2018 (the third FDA clearance for Photonica USA), offering the most effective noninvasive cellulite treatment.
Working together with Canfield Scientific, in 2019 we developed state-of-the-art 3D imaging technology for automated measurements and volumetric analysis, showing patients their immediate fat loss with UltraSlim®.
Constant Quality Improvement means we can never be satisfied, even with the safest and most effective devices for fat, skin, and cellulite. During the pandemic of 2020, we became FDA_GMP-certified, meeting the world’s highest quality standards for medical device manufacturers even as our engineering team was busy making dramatic scientific breakthroughs in:
- High output diode cooling systems,
- Internet of Things cybersecurity,
- Remote digital control systems accessible from any Internet browser, and
- The server-based Photonica® App for Apple and Android mobile devices and a desktop version for web browsers running the server app at PhotonicResearch.com.
In December 2020, we received FDA clearance K202361 for our "Internet of Things" (IoT) approach to remote control of our first-generation "UltraSlim® Digital" and "UltraSmooth® Digital" devices.
Also in December 2020, all business assets of medical device manufacturer Vevazz, LLC were acquired by Ward Photonics.
In 2023, we implemented Bluetooth connectivity as a second-generation alternative to Internet access.
Also in 2023, all business assets of Ward Photonics LLC were acquired by Photonica USA, LLC.
Today, Photonica USA manufactures best-in-class medical devices and 3D imaging systems under authorization and license from Blue Water Innovations, LLC, which is the owner of all accreditations, patents, trademarks, service marks, copyrights, designs, schematics, research, FDA clearances, and other intellectual property.
IRB Clinical Trials for FDA: Fat Reduction
In 2016 multi-site clinical trials (NCT02867150 at ClinicalTrials.gov), researchers documented that UltraSlim® provides the results that so many of your patients want:
- Immediate Results. All patients lost fat immediately -- without dieting, exercise, drugs, or surgery.
- Dramatic Fat Loss At Each Visit. Patients lost an average of 1,580cc of fat in just 32 minutes (3.5" combined waist, hips, and thighs). During one visit!
- Works For Everyone. Every patient had clinically significant losses at each visit, ranging from 717cc to 4,608cc (1 5/8" to 10" from the waist, hips, and thighs).
- Durability. Patients seen at follow-up had lost an additional 1 1/8" of fat during the week following treatment. With UltraSlim®, patients lose the fat permanently through lipolysis, whereby the contents of the fat cells are excreted primarily in their waste.
IRB Clinical Trials for FDA: Cellulite and Fat Reduction
In 2017 multi-site clinical trials (NCT03647748 at ClinicalTrials.gov), researchers documented that UltraSmooth® is second only to UltraSlim® for fat reduction (average losses of 3.9" 14 days post-treatment) and UltraSmooth® also significantly improves the appearance of cellulite noninvasively.
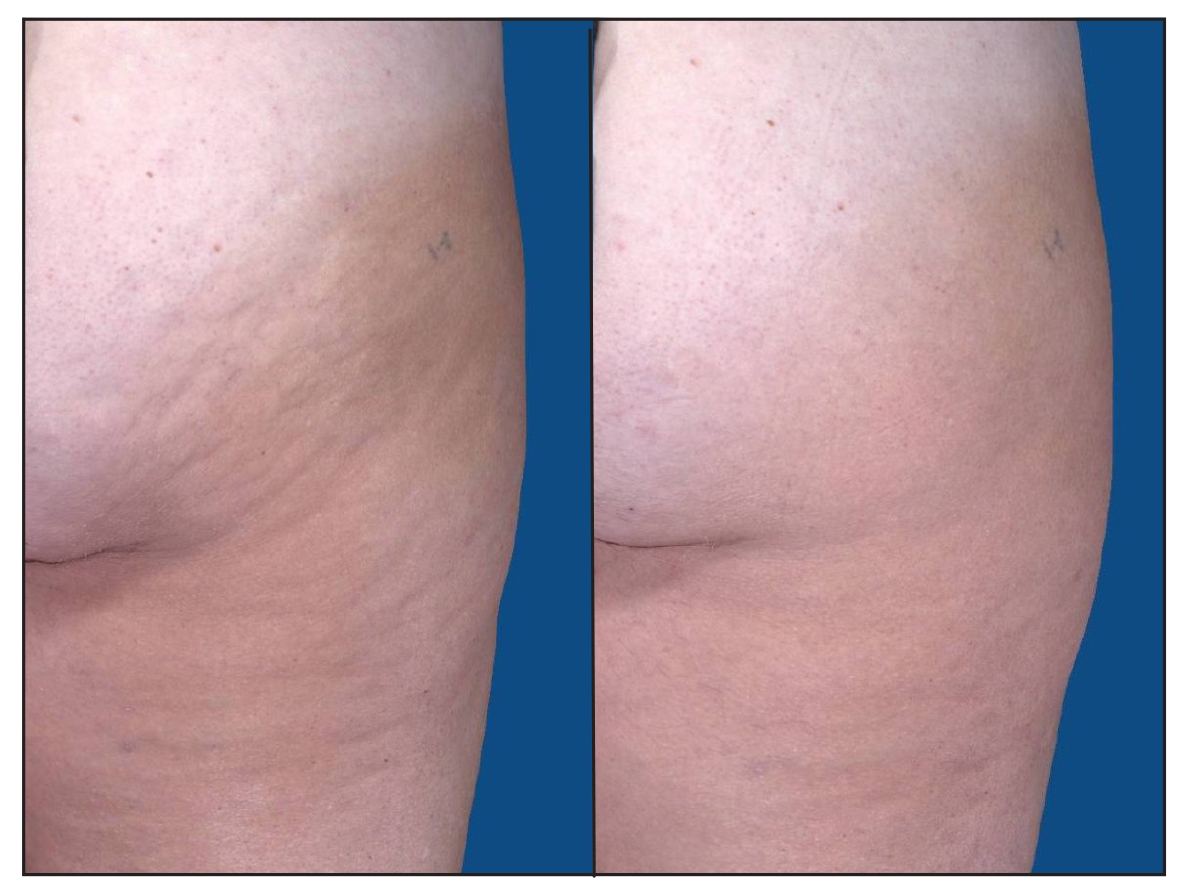
Clinical Studies
Listed below are studies demonstrating the power, penetration, and efficacy of UltraSlim®, along with blood chemistry studies which find no increase in serum triglycerides or cholesterol:
- 2012 Comparison of UltraSlim® and Laser Transmission Through Skin and Fat
- 2016 IRB Clinical Trials NCT02867150 at ClinicalTrials.gov
- 2017 Intra-Surgical Study of Fat Reduction In Vivo
- 2017 UltraSmooth® IRB Clinical Trials NCT03647748 at ClinicalTrials.gov
- 2019 Blood Chemistry Studies by Drs. Ruth and Olaf Rustad
- 2021 International Society of Aesthetic Plastic Surgery, Clinical Trials by Robert H. Burke, MD, MS, DDS, FACS, FICS
- IRB Clinical Trials: Effects of a Red/Gold/IR LED Combination Light on Reduction of Fat. We are currently sponsoring clinical trials (NCT04525573 at ClinicalTrials.gov) as an open-label evaluation of the effects of a red/gold/IR LED combination light on the reduction of fat. The results shall be compared to previous results from a comparator UltraSlim® device utilizing only red LED monotherapy for the non-invasive reduction in fat layer for body contouring.
- IRB Clinical Trials: Treatment of Lung Inflammation. We are currently sponsoring clinical trials (NCT04524715 at ClinicalTrials.gov) to determine if a reduction of pneumonic inflammation occurs after treatment with our investigational device applying red-light technology in the respiratory system of patients suffering from acute viral pneumonia.
- The United States Patent and Trademark Office has also accepted our U.S. Patent Application No.: 63/074,790 for the treatment of respiratory diseases and more generally a utility patent for RESPIRATORY TREATMENT DEVICE AND METHOD UTILIZING OPTICAL EMITTERS, as invented by Judson Ward and CEO Terry J. Ward, Sr., M.H.A.
- Novel research combining UltraSlim® and UltraSmooth® for the treatment of lymphedema by vascular surgeon Michael Romberg, MD, FACS is on-going.
- Novel research on UltraSlim® for the treatment of surgical wounds to accelerate healing by investigator Robert H. Burke, MD, MS, DDS, FACS, FICS is on-going.

